Description
Description
(USP 44)
- Drug Substance General Information (ICH 3.2.S.1)
1.1. Nomenclature (ICH 3.2.S.1.1)
International non-proprietary name: Montelukast Sodium (Brand Name: Singulair)
Compendial name: Montelukast Sodium
Chemical name: [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(hydroxymethylethyl)phenyl]propyl]thio]methyl]-cyclopropane acetic acid, monosodium salt.
Arasto’s code: MON
CAS Registry Number: CAS [151767-02-1]
- Drug Substance General Information (ICH 3.2.S.1)
1.2. Structure (ICH 3.2.S.1.2)

Empirical formula: C35H35ClNNaO3S
Molecular Weight: 608.18 g/mol
- Drug Substance General Information (ICH 3.2.S.1)
1.3. General Properties (ICH 3.2.S.1.3)
Montelukast Sodium is a white to off-white powder. It is administered orally for prophylaxis and chronic treatment of asthma, exercise-induced bronchoconstriction, and allergic rhinitis. It is soluble in water and most organic solvents except saturated hydrocarbons. Estimated pKa of montelukast is 4.3 (http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO +7582) and its calculated log P has been reported to be 8.98 (http://www.ncbi.nlm.nih. gov/pmc/articles/PMC2641037/). Montelukast sodium is unstable in acid and base and undergoes oxidation at the sulfur tom to afford a sulfoxide impurity. It can also undergo light-induced double bond isomerization (see Stability Studies). Oral LD50 for Montelukast Sodium in rat has been reported to be>5000 mg/kg (http://www.lclabs.com/MSDS/M-4966MSDS.php4).
The determination of purity and assay of APIs require comparison of the product with their respective Reference Standards (RS) and Related Compounds (RC or known impurities). Accordingly, ICH regulations on the purity and assay of reference standard and related compounds are clearly defined and must be followed by drug substance and drug product manufacturers.
According to ICH Q7, 11.1 there are 3 types of standards. This is summarized in the following chart and discussed in detail below.

The impurities provided in the following table represent Secondary Reference Standards (SRS) that are prepared in-house by synthesis or by isolation. Each SRS has undergone extensive characterization (IR, UV, 1HNMR, 13CNMR. Mass Spec) and determination of its purity and assay (HPLC). For specification of the SRS of those products that have a monograph, the SRS is compared with a pharmacopoeia Primary Reference Standard (UV, HPLC retention time). For specification of those products that do not have a monograph (known as House Primary Standard), we compare their UV ε or ג/max, IR major absorptions, 1HNMR d (ppm), 13CNMR d (ppm) or HPLC retention time with values reported in the chemical literature for these compounds.
| Structure | Chemical Name | USP Code |
 | Dicyclohexylammonium (RS,E)-2-(1-{[(1-{3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl}-3-[2-(2-hydroxypropan-2- yl)phenyl]propyl)thio]methyl}cyclopropyl)acetate | Monelukast Racemate |
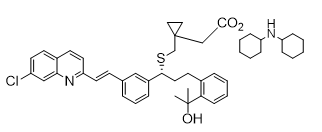 | Dicyclohexylammonium (R,E)-2-(1-{[(1-{3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl}-3-[2-(2- hydroxypropan-2-yl)phenyl]propyl)thio]methyl}cyclopropyl)acetate | Montelukast Dicyclohexylamine |
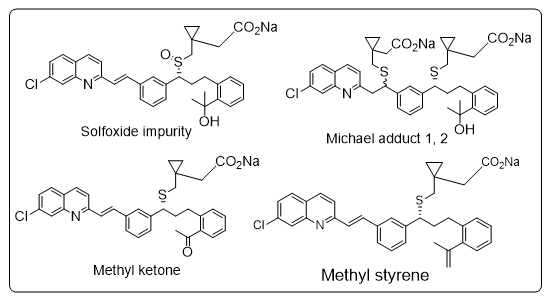 | Montelukast containing sulfoxide impurity, michael adducts 1 and 2, methylketone impurity, and methylstyrene impurity | Montelukast for Peak Identification |
Montelukast sodium Related Compounds
- Primary and Secondary Reference Standard (ICH 3.2.S.5)
5.1. Active Pharmaceutical Ingredient
Primary Reference Standard for montelukast sodium is available from USP. We will use a Secondary Reference Standard (previously referred to as Working Standard) for direct control of all batches of montelukast sodium.
As per ICH (Q7, 11.1) and ICH (Q6, 2.11, 3.2, 3.3) the Secondary Reference Standards, which include the API and its Related Compounds, must be examined for their proof of structure (characterization), assay and purity and specification (identification by comparison). Furthermore, ICH Guideline on the Preparation of Common Technical Document (Q4M) requires that the data obtained from characterization, assay and purity and specification must be included in section 3.2.S.3.2 for Related Compounds (already discussed in that section) and section 3.2.S.5 of the DMF for the API. To this end, the Secondary Reference Standards of the API montelukast sodium has undergone extensive characterization (UV, IR, 1 H NMR, 13C NMR, and Mass Spec) to assure its structure, assay and purity (HPLC and/or titration) and specification (comparison of its HPLC retention time and UV ג /max with USP Primary Reference Standard.
The Secondary Reference Standard for montelukast sodium was produced from a released batch of montelukast sodium by subjecting it to an additional crystallization from the final solvent system used in the production of the API to avoid the possibility of other polymorph formation.
SPECIFICATION OF ANALYSIS
| Product: Montelukast Sodium | CAS No.: 151767-02-1 | Spec. No.: APC-QC-SPEC-385-00 | ||
| Issue Date: Apr, 2023 | Valid up to: Apr, 2024 | Reference: USP44 | ||
| Tests | Specifications | |||
| Description | White or almost white, hygroscopic powder. | |||
| Solubility | Freely soluble to very soluble in alcohol; freely soluble in water and in methylene chloride. | |||
| Identification | A: Infrared Spectroscopy B: Sodium Test C: Enantiomeric purity | |||
| Water Determination | NMT 4.0% | |||
| Enantiomeric Purity | NMT 0.2% of The S-enantiomer | |||
| Residual Solvents | N-heptane: NMT 5000 ppm (Class Ш) Toluene: NMT 890 ppm (Class II) | |||
| Organic Impurities (HPLC) | Sulfoxide impurity: NMT 0.2% Cis-isomer: NMT 0.15% Michael Adducts 1cand 2d : NMT 0.15% Methylketone impurity: NMT 0.15% Methylstyrene impurity: NMT 0.3 % Any other individual impurity: NMT 0.10% Total impurities: NMT 0.6 % | |||
| Assay (HPLC) | 98.0% to 102.0 % (on the anhydrous and solvent-free basis). | |||
| Prepared by: M. Shahbazi, B.Sc.Chem. | Checked by: A. Forghani, B.Sc.Chem. | |||
| Approved by: F. Javadizadeh, M.Sc.Chem. | ||||
| Storage: Preserve in tight containers, protected from light. Store at room temperature. | ||||





