Description
Description
(USP 44)
- Drug Substance General Information (ICH 3.2.S.1)
1.1. Nomenclature (ICH 3.2.S.1.1)
International non-proprietary name: Aprepitant (Brand Name: Emend)
Compendial name: Aprepitant
Chemical name: 5-([(2R,3S)-2-((R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy)-3-(4-fluorophenyl)morpholino]methyl)-1H-1,2,4-triazol-3(2H)-one.
Arasto’s code: APR
CAS Registry Number: [170729-80-3]
- Drug Substance General Information (ICH 3.2.S.1)
1.2. Structure (ICH 3.2.S.1.2)
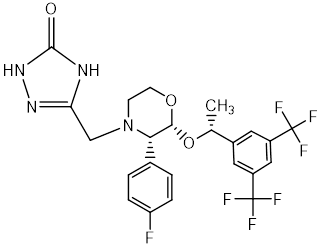
Empirical Formula: C23H21F7N4O3
Molecular Weight: 534.427 g/mol
- Drug Substance General Information (ICH 3.2.S.1 )
1.3. General Properties (ICH 3.2.S.1.3)
Aprepitant is an off-white crystalline solid that has a molecular weight of around 534.53. It has a very limited solubility in water. It does have a reasonably high solubility in non-polar molecules such as oils. This would, therefore, suggest that aprepitant as a whole, despite having components that are polar, is a non-polar substance. Aprepitant is manufactured by Merck & Co. under the brand name Emend for prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) and for prevention of postoperative nausea and vomiting. It was approved by the FDA in 2003.
The determination of purity and assay of APIs require comparison of the product with their respective Reference Standards (RS) and Related Compounds (RC or known impurities). Accordingly, ICH regulations on the purity and assay of reference standard and related compounds are clearly defined and must be followed by drug substance and drug product manufacturers.
According to ICH Q7, 11.1 there are 3 types of standards. This is summarized in the following chart and discussed in detail below.
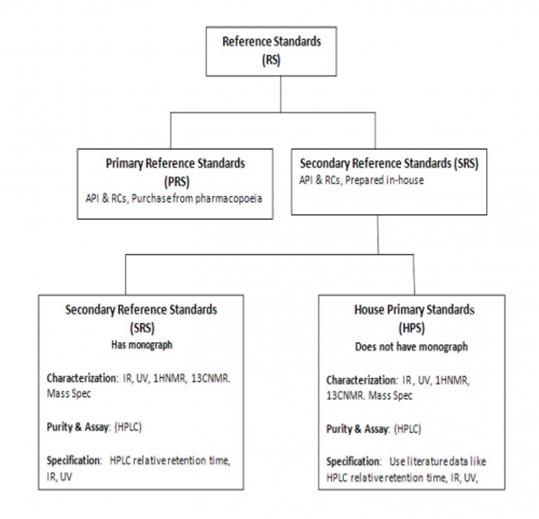
The impurities provided in the following table represent Secondary Reference Standards (SRS) that are prepared in-house by synthesis or by isolation. Each SRS has undergone extensive characterization ( IR, UV, 1HNMR, 13CNMR. Mass Spec) and determination of its purity and assay (HPLC). For specification of the SRS of those products that have a monograph, the SRS is compared with a pharmacopoeia Primary Reference Standard (UV, HPLC retention time). For specification of those products that do not have a monograph (known as House Primary Standard), we compare their UV ε or ג /max , IR major absorptions, 1HNMR d (ppm) , 13 CNMR d (ppm) or HPLC retention time with values reported in the chemical literature for these compounds.
Aprepitant Related Compounds
| Structure | Chemical Name | USP Code | Arasto Code |
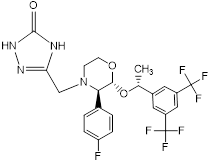 |
3-[[(2R,3R)-2-[(R)-1-[3,5-bis(trifluo romethyl)phenyl]ethoxy]-3-(4-fluoro phenyl)morpholino]methyl]-1H-1,2, 4-triazol-5(4H)-one. (R,R,R-Diastereomer). |
RCA | RCA |
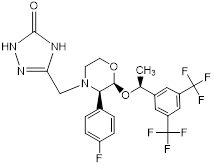 | 3-[[(2S,3R)-2-[(S)-1-[3,5- bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholino] methyl]-1H-1,2,4-triazol-5(4H)-one. (S,R,S-Enantiomer) | RCB | RCB |
 | 5-[[(2R,3S)-2-[(R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-phenylmorpholino]methyl]-2H-1,2 ,4-triazol-3(4H)-one. (Desfluoro Aprepitant) | Desfluoro aprepitant | Desfluoro aprepitant |
- Primary and Secondary Reference Standard (ICH 3.2.S.5)
5.1. Active Pharmaceutical Ingredient
Primary Reference Standard for Aprepitant is available from United States Pharmacopoeia. We will use a Secondary Reference Standard (previously referred to as Working Standard) for direct control of all batches of Aprepitant.
As per ICH (Q7, 11.1) and ICH (Q6, 2.11, 3.2, 3.3) House Primary Standards, which include the API and its Related Compounds, must be examined for their proof of structure (characterization), assay and purity and specification (identification by comparison). Furthermore, ICH Guideline on the Preparation of Common Technical Document (Q4M) requires that the data obtained from characterization, assay and purity and specification must be included in section 3.2.S.3.2 for Related Compounds (already discussed in that section) and section 3.2.S.5 of the DMF for the API. To this end, the House Primary Standard of the API Aprepitant has undergone extensive characterization (UV, IR, 1 H NMR, 13C NMR, Mass Spec) to assure its structure, assay and purity (HPLC and/or titration) and specification.
The Secondary Reference Standard for Aprepitant was produced from a released batch of aprepitant by subjecting it to an additional crystallization from the final solvent system used in the production of the API to avoid the possibility of other polymorph formation.
SPECIFICATION OF ANALYSIS
| Product: Aprepitant | CAS No.: 170729-80-3 | Spec. No.: APC-QC-SPEC-370-00 | ||
| Issue Date: Apr, 2023 | Valid up to: Apr, 2024 | Reference: USP44 | ||
| Tests | Specifications | |||
| Description | White to off-white powder. | |||
| Solubility | Sparingly soluble in alcohol; slightly Soluble in acetonitrile; practically insoluble in water. | |||
| Identification | A: Infrared Spectroscopy B: The retention of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. | |||
| Residue on ignition | NMT 0.1% | |||
| Specific rotation | +66.0° to +71.0° (10mg/ml in Methanol) | |||
| Water determination | NMT 0.5% | |||
| Limit of S,R,S-Enantiomer (HPLC) | NMT 0.10% of the S,R,S-enantiomer | |||
| Residual Solvents | Methanol: Max. 3000 ppm (Class II) Isopropanol: Max. 5000 ppm (Class III) | |||
| Organic impurities (HPLC) | Desfluoro Aprepitant: NMT 0.15% Any individual unspecified impurity: NMT 0.10% Total impurities: NMT 0.30% | |||
| Assay (HPLC) | 98.0% to 102.0% (on the anhydrous and solvent-free basis) | |||
| Prepared by: M. Shahbazi, B.Sc.Chem. | Checked by: A. Forghani, B.Sc.Chem. | |||
| Approved by: F. Javadizadeh, M.Sc.Chem. | ||||
| Storage: Preserve in well-closed containers, protected from light. Store at room temperature. | ||||





