Description
Description
(USP44)
Description
- Drug Substance General Information (ICH 3.2.S.1)
1.1. Nomenclature (ICH 3.2.S.1.1)
International Non-proprietary Name: Paclitaxel (Brand Name: Taxol)
Compendial Name: Paclitaxel
Chemical Name: 5β, 20-Epoxy-1,2α,4,7β,10β,13α- hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester
Arasto’s Code: PAC
CAS Registry Number: [33069-62-4]
Official Pharmacopoeia Monograph: USP 44
1. Drug Substance General Information (ICH 3.2.S.1)
1.2. Structure (ICH 3.2.S.1.2)
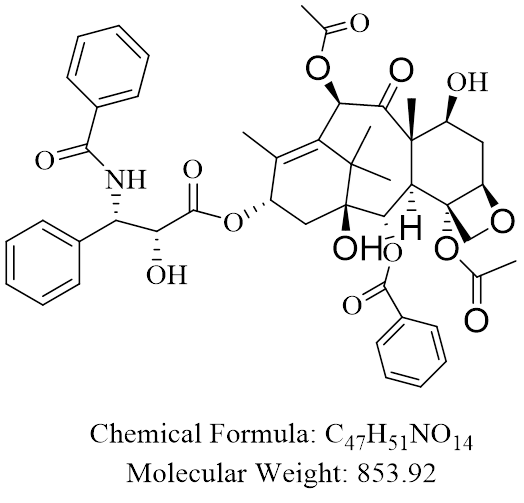
- Drug Substance General Information (ICH 3.2.S.1 )
1.3. General Properties (ICH 3.2.S.1.3)
Paclitaxel is a natural product with antitumor activity. Paclitaxel is obtained via a semi-synthetic process from Taxus baccata. The chemical name for paclitaxel is 5β, 20-Epoxy-1,2α,4,7β,10β,13α- hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine. Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and a molecular weight of 853.9. It is highly lipophilic, insoluble in water, and melts at around 216-217° C.
The determination of purity and assay of APIs require comparison of the product with their respective Reference Standards (RS) and Related Compounds (RC or known impurities). Accordingly, ICH regulations on the purity and assay of reference standard and related compounds are clearly defined and must be followed by drug substance and drug product manufacturers.
According to ICH Q7, 11.1 there are 3 types of standards. This is summarized in the following chart and discussed in detail below.

The impurities provided in the following table represent Secondary Reference Standards (SRS) that are prepared in-house by synthesis or by isolation. Each SRS has undergone extensive characterization (IR, UV, 1HNMR, 13CNMR. Mass Spec) and determination of its purity and assay (HPLC). For specification of the SRS of those products that have a monograph, the SRS is compared with a pharmacopoeia Primary Reference Standard (UV, HPLC retention time). For specification of those products that do not have a monograph (known as House Primary Standard), we compare their UV ε or ג /max, IR major absorptions, 1HNMR d (ppm), 13CNMR d (ppm) or HPLC retention time with values reported in the chemical literature for these compounds.
Paclitaxel USP Related Compounds
| Structure | Chemical name | USP Code | Arasto Code |
| Cephalomannine | Paclitaxel related compound A | Paclitaxel related compound A | |
| 10-Deacetyl-7-epipaclitaxel | Paclitaxel related compound B | Paclitaxel related compound B |
- Primary and Secondary Reference Standard (ICH 3.2.S.5)
5.1. Active Pharmaceutical Ingredient
Primary Reference Standard for Paclitaxel is available from United States Pharmacopoeia. We will use a House Primary Standard (previously referred to as Working Standard) for direct control of all batches of Paclitaxel.
As per ICH (Q7, 11.1) and ICH (Q6, 2.11, 3.2, 3.3) House Primary Standards, which include the API and its Related Compounds, must be examined for their proof of structure (characterization), assay and purity and specification (identification by comparison). Furthermore, ICH Guideline on the Preparation of Common Technical Document (Q4M) requires that the data obtained from characterization, assay and purity and specification must be included in section 3.2.S.3.2 for Related Compounds (already discussed in that section) and section 3.2.S.5 of the DMF for the API. To this end, the House Primary Standard of the API Paclitaxel has undergone extensive characterization (UV, IR, 1 H NMR, 13C NMR, Mass Spec) to assure its structure, assay and purity (HPLC and/or titration) and specification.
The House Primary Standard for Paclitaxel was produced from a released batch of Paclitaxel by subjecting it to an additional crystallization from the final solvent system used in the production of the API to avoid the possibility of other polymorph formation. It was crystallized twice more to ensure high level of purity.
SPECIFICATION OF ANALYSIS
| Product: Paclitaxel | CAS No.: 33069-62-4 | Spec. No.: APC-QC-SPEC-361-00 | ||
| Issue Date: Apr, 2023 | Valid up to: Apr, 2024 | Reference: USP44 | ||
| Tests | Specifications | |||
| Description | White to off- white powder. | |||
| Solubility | Soluble in alcohol; insoluble in water. | |||
| Identification | A: Infrared spectroscopy B: The retention of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation, as obtained in the Assay. | |||
| Specific Rotation | Between -49.0° and -55.0°, at 20° calculated on the anhydrous, solvent-free basis (10mg/ml in methanol) | |||
| Water determination | NMT 4.0% | |||
| Residue on ignition | NMT 0.2% | |||
| Related compounds, Test 2 | 10-Deacetylbaccatin III : NMT 0.1% Baccatin III : NMT 0.2% Photodegradant: NMT 0.1% 10-Deacetylpaclitaxel : NMT 0.5% 2-Debenzoyl paclitaxel-2-Pentenoate: NMT 0.7% Oxetane ring opened, acetyl and benzoyl migrated: x1 10-Acetoacetylpaclitaxel : x2 10-Deacetyl-7-epipaclitaxel (paclitaxel related compound B): x3 The sum of x1, x2, x3 is not more than 0.4% 7-Epipaclitaxel: NMT 0.4% 10, 13-Bissidechainpaclitaxel: NMT 0.5% 7-Acetylpaclitaxel: NMT 0.6% 13-Tes-baccatin III : NMT 0.1% 7-Tes-paclitaxel: NMT 0.3% Any other single impurity: 0.1% Total impurities: NMT 2.0% | |||
| Residual solvents | Acetone: NMT 5000 ppm (Class III) Dichloromethane: NMT 600 ppm (Class II) Acetonitrile: NMT 410 ppm (Class II) n-heptane: NMT 5000 ppm (Class III) Toluene: NMT 890 ppm (Class II) | |||
| Assay | 97.0% to 102.0% (on the anhydrous, solvent-free basis) | |||
| Bacterial Endotoxins test | NMT 0.4 USP Endotoxin Unit per mg of paclitaxel. | |||
| Microbial enumeration tests and Tests for specified microorganisms | The total aerobic microbial count does not exceed 100 cfu/g. It meets the requirements of the tests for the absence of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella species, and Escherichia coli. | |||
| Prepared by: M. Shahbazi, B.Sc.Chem. | Checked by: A. Forghani, B.Sc.Chem. | |||
| Approved by: F. Javadizadeh, M.Sc.Chem. | ||||
| Storage: Preserve in tight, light-resistant containers, and store at controlled room temperature. | ||||